In the previous lesson we started our discussion of structures with more than one ring, using decalin as our key example of a fused ring. We saw how much the stereochemistry at the ring junction can affect the overall shape of the molecule, as well as its stability.
1. Fused Rings, Bridged Bicyclic Rings, And Spiro Rings
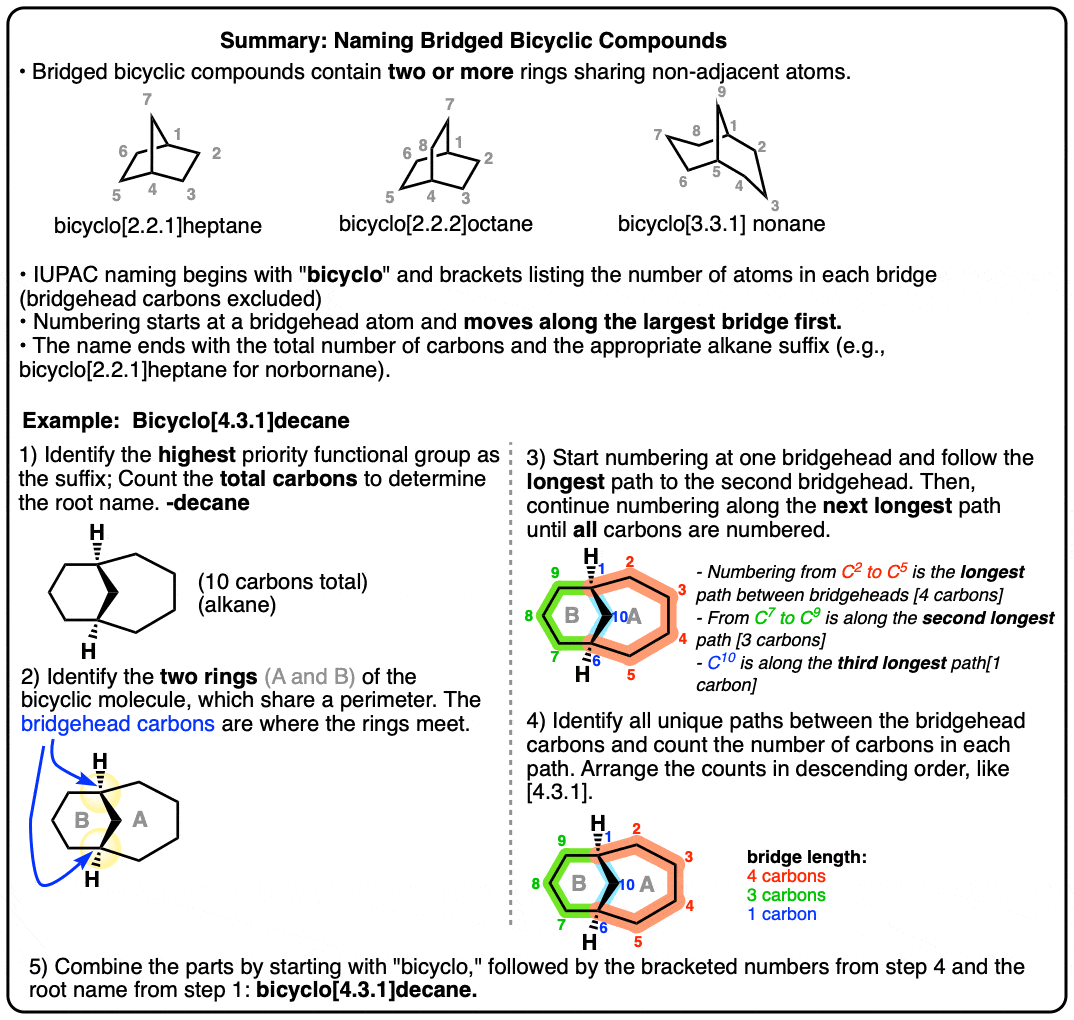
What we didn’t talk about is that the ring junction of decalin represents only one way to arrange the ten carbons of decalin into a pair of adjacent rings: the so-called “fused” ring structure, where the two “bridgehead” carbons are directly connected.
There are actually two other modes of ring junction. In “bridged bicyclic” molecules, the two bridgeheads are separated by “bridges” containing at least one carbon. In “spiro” fused molecules, the two rings are both joined at the same carbon.
In this lesson we’ll focus on “bridged bicyclic” molecules (and how to name them), and also briefly touch on “spiro” fused bicyclic molecules.
By the way, in the diagram below, only one “bridged bicyclic” isomer of decalin with a six-membered ring is drawn. Can you find the other bridged bicyclic isomer that also contains a six-membered ring? (Answer in Note 1)
2. In Or Out? Either Both In, Or Both Out.
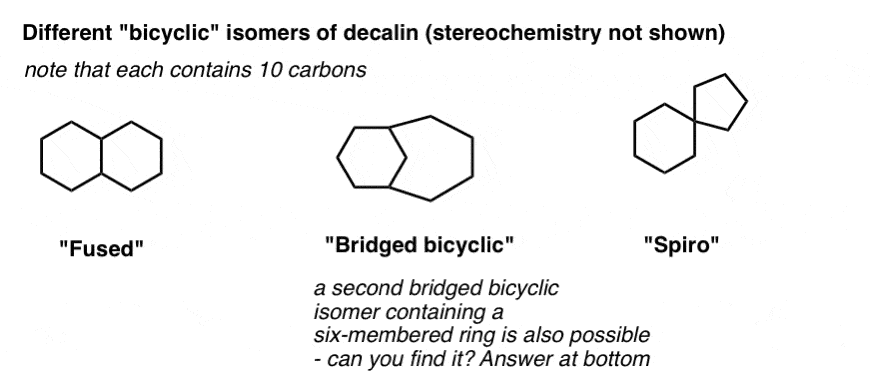
First, stereochemistry. One important note about bridged bicyclic molecules is that the two carbons forming each bridge will always be “cis” to each other, never trans, relative to the other ring.* [exceptions? yes, but you probably won’t see ’em – check Note 2 if you’re curious]
Why? Like we said in the last lesson when discussing trans-decalin, for much the same reason that you can’t kiss yourself on the back – there just isn’t enough flexibility for this to happen without breaking something. Given the strict bond angle (109°) and length (1.50 Å) requirements of alkanes, five, six, and seven membered rings don’t have enough slack to tolerate anything other than cis ring junctions. A trans ring junction (much like a trans double bond in rings of these sizes) would simply lead to too much ring strain.
Note that in the diagram below, the “cis” ring junctions is implied by the fact that both hydrogens are on the same side (both dashes in this case). The “impossible” trans ring junction is shown in grayscale for comparison. The picture shows a model of this molecule highlighting the angle strain and Van Der Waals strain resulting from this arrangement.
3. Putting Bridged Compounds In Perspective
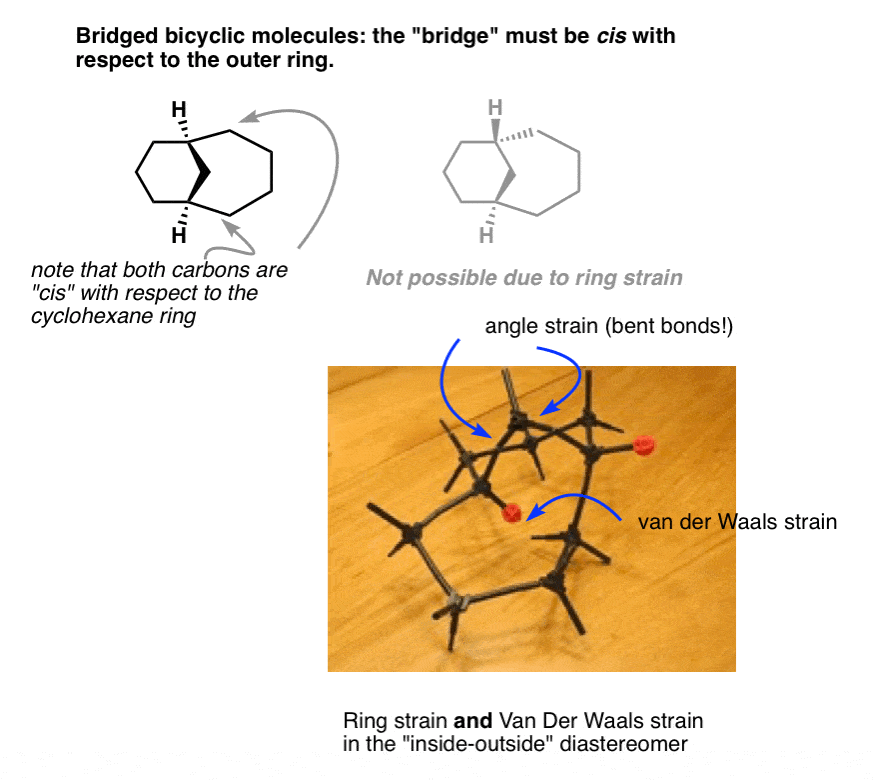
Next – and this is where a lot of students get confused – we come to the topic of how to depict these things. Merely using dashes and wedges doesn’t truly capture their three-dimensional beauty.
So when drawing bridged bicyclic molecules, it’s very common to show them in perspective from the side. The first reaction my students have upon seeing these drawings is utter disgust and confusion. “What is THAT?”, I recall Mike from Sault Ste. Marie asking me one night during a tutoring session.
It’s the same thing as the “top down” view, just drawn from a different perspective. Using the “top down” view is a perfectly acceptable way to draw these molecules – however, it’s vital to be able to interpret these “perspective” drawings as many bicyclic molecules in your textbook will be shown in this way.
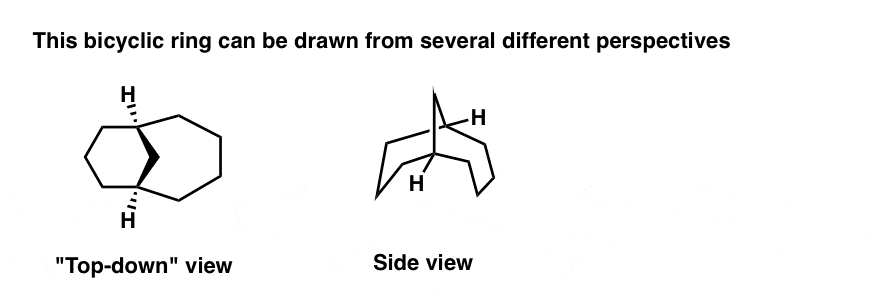
Here’s a “flyover” view of the same molecule, from top-down to the side-view. [BTW: note how the six membered ring on the left is in the chair conformation]
4. Naming Bridged Bicyclic Molecules – A Five-Step Guide
The naming of bridged bicycles has its own special kind of funk. Unlike the molecules you’ve likely come across so far, which will have a clear “longest chain” or “largest ring” to start from, trying to find the place to start based on those criteria alone will likely have you going in circles.
Instead, bridged bicycles are named according to a unique system of their own. based on the length of their bridges, and then the overall number of carbons in the bicycle. The figure below walks through the process.
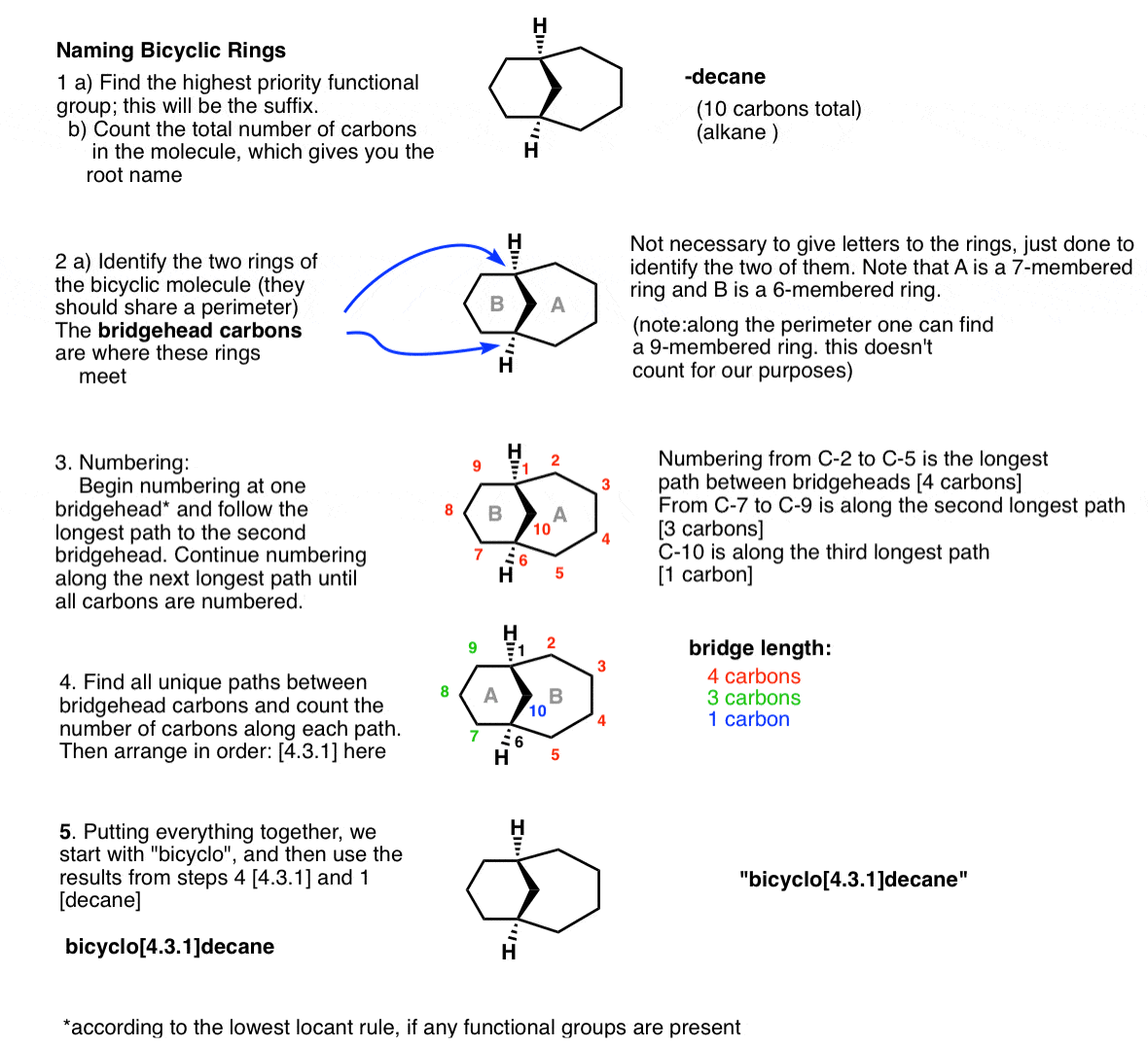
5. A Few More Examples Of Bicyclic Molecules
Once you’ve run through a few examples by yourself, I think you’ll find that naming bridged bicyclic molecules is actually fairly intuitive – as long as you can interpret the diagrams correctly. [Try making a model if you’re still stuck!] See if you can follow the naming of these compounds.
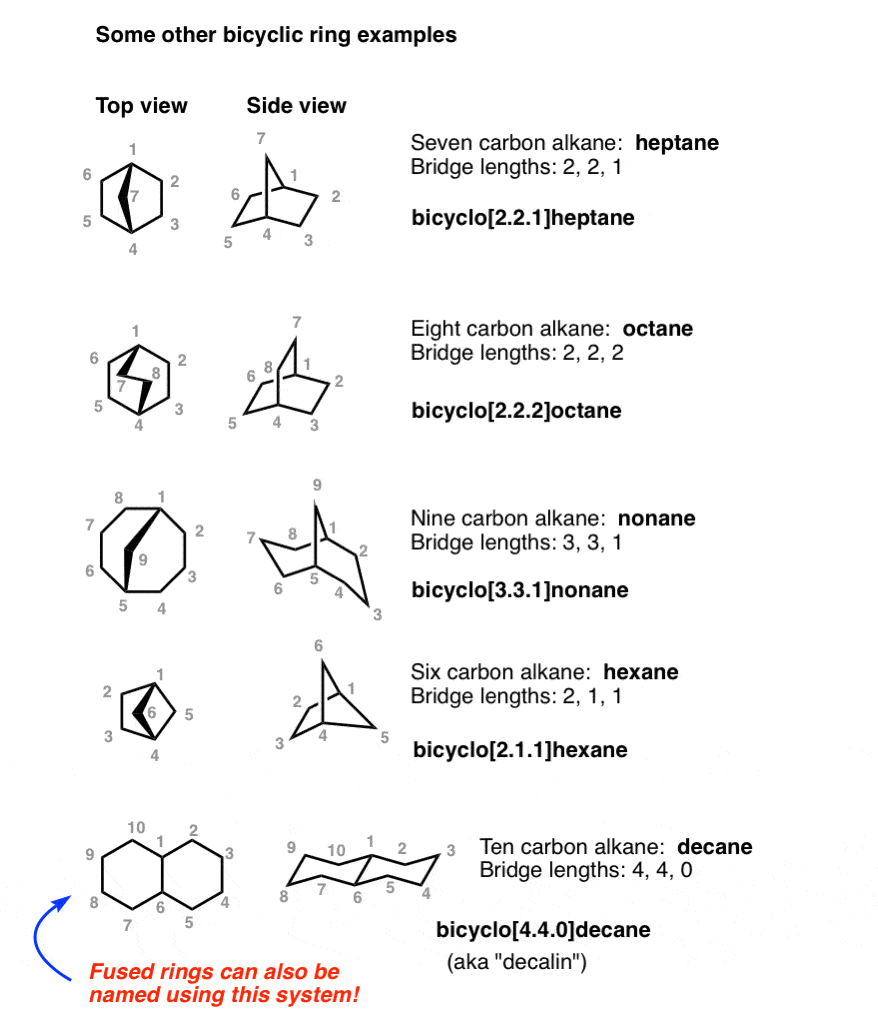
See that last example? We can also use bridged bicycle nomenclature to name fused rings as well! So bicyclo[4.4.0]decane is simply another name for “decalin” (without specifying the stereochemistry, of course).
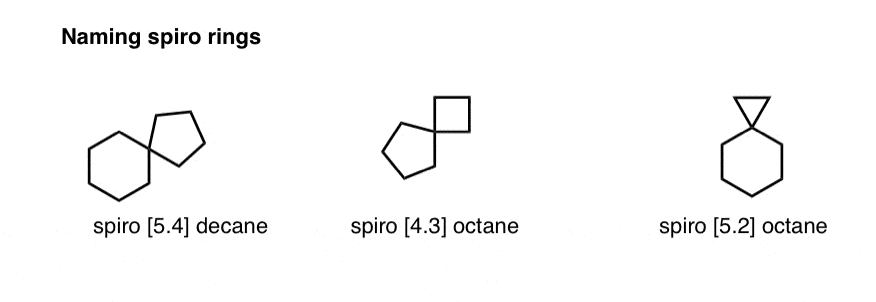
6. Naming Spiro Compounds
Let’s wrap up by briefly covering “spiro” fused compounds. Since both “bridgehead” positions are on the same carbon, we won’t be able to use the same “bicyclo” nomenclature as before- but the process is very similar.
We simply substitute “spiro” for “bicyclo” , insert the two bridge lengths, and place the suffix as before. So the molecule below is spiro[5.4]decane. Included next door are two other examples of spiro compounds, spiro[4.3]octane and spiro[5.2]octane.
7. Summary: Naming Bicyclic Compounds
In the next lesson – and last in this series – we’ll talk about one final, very interesting consequence of the fact that carbons can form rings: Bredt’s Rule.
Leave a Reply